The brightest example of fusion reactions in nature is the Sun and the stars in which hydrogen nuclei, bound together by strong gravitational force, fuse in a chain of reactions shown in Table 1 to produce finally Helium.
| Table 1 : Fusion reactions in the Sun | |||||
|---|---|---|---|---|---|
| p+p | D | + | e+ | First two protons (hydrogen nuclei) fuse to produce a deuterium and a positron | |
| p+D | He3 | + | gamma Radiation |
A proton and a deuterium fuse to produce a light isotope of Helium, He3 (He with one neutron) | |
| He3+He3 | He4 | + | D+D | Two He3 fuses to produce He4 (He with 2 neutrons), for each of this reaction to happen, the first two reactions have to occur twice | |
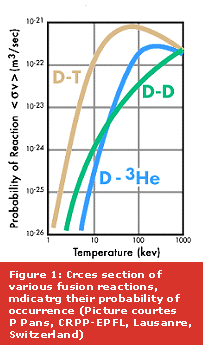
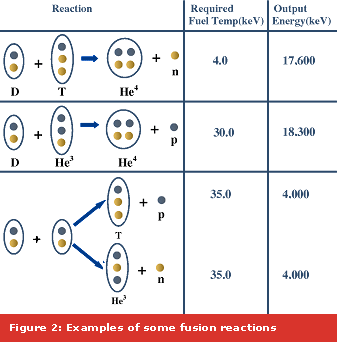
In laboratory experiments, the fusion reaction that is least difficult to achieve is that between a deuterium and a tritium nucleus, because it is the one that occurs at relatively lower temperatures, as is evident from the various reaction probabilities as shown in Figure 1. However, several fusion reactions are possible between the lightest of nuclei, namely hydrogen (H) and its isotopes (Deuterium (D) and Tritium (T) with one and two extra neutrons respectively) isotope of helium, He3 or even boron (B), Lithium (Li) and so on.. Some of these reactions and their products are shown in Figure 3.
Unlike in the Sun, where particles are tightly bound together by the enormous gravitational pull of the huge mass of the Sun, on earth, it takes enormous effort to keep the charged particles together (note that like charges repel). This is the major area of fusion research - that of confinement, i.e., how to maintain a plasma of large enough density of Deuterium and Tritium at a high enough temperature for a sufficiently long time so that the particles fuse and produce net energy, larger than that required to produce and hold the plasma in the first place.